More than 500 million people worldwide suffer from type 2 diabetes (T2D), one of the chronic illnesses with the fastest rate of growth in our time. It is defined by the loss of insulin-producing beta cells in the pancreas and the body's increasing incapacity to control blood glucose as a result of insulin resistance.
Insulin secretion becomes inadequate as the disease progresses due to the degeneration of pancreatic beta cells as well as cell resistance. Long-term care becomes more challenging due to this dual burden, which also causes serious side effects like kidney failure, neuropathy, cardiovascular disease, and vision loss.
However, recent advances in peptide-based biotechnology are reviving hope among scientists and patients. Researchers are looking into ways to preserve and regenerate the beta cells themselves, which would restore the body's natural production of insulin, as opposed to merely replacing insulin or lowering blood sugar.
Understanding the Problem: Inflammation and Beta Cell Loss
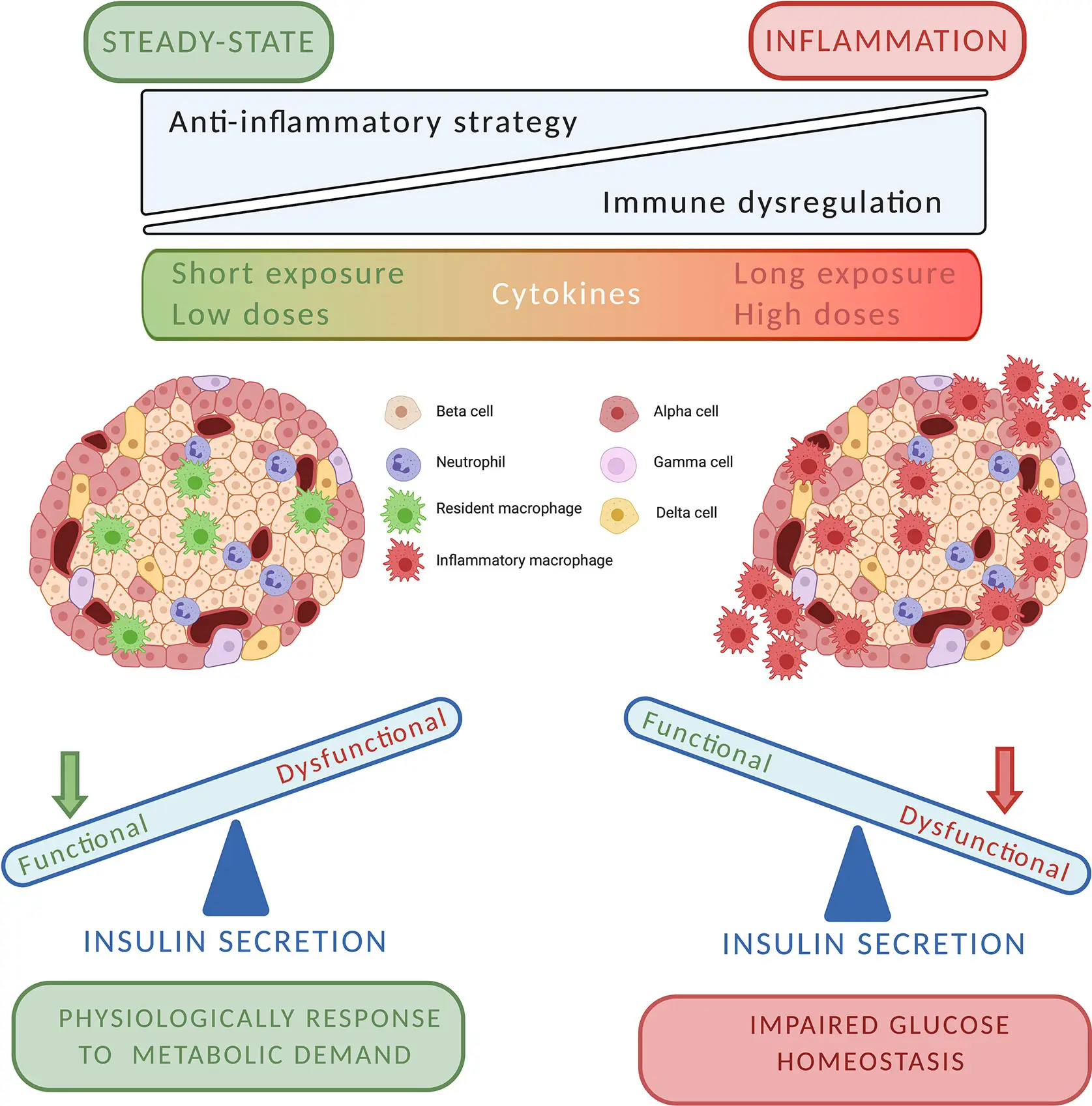
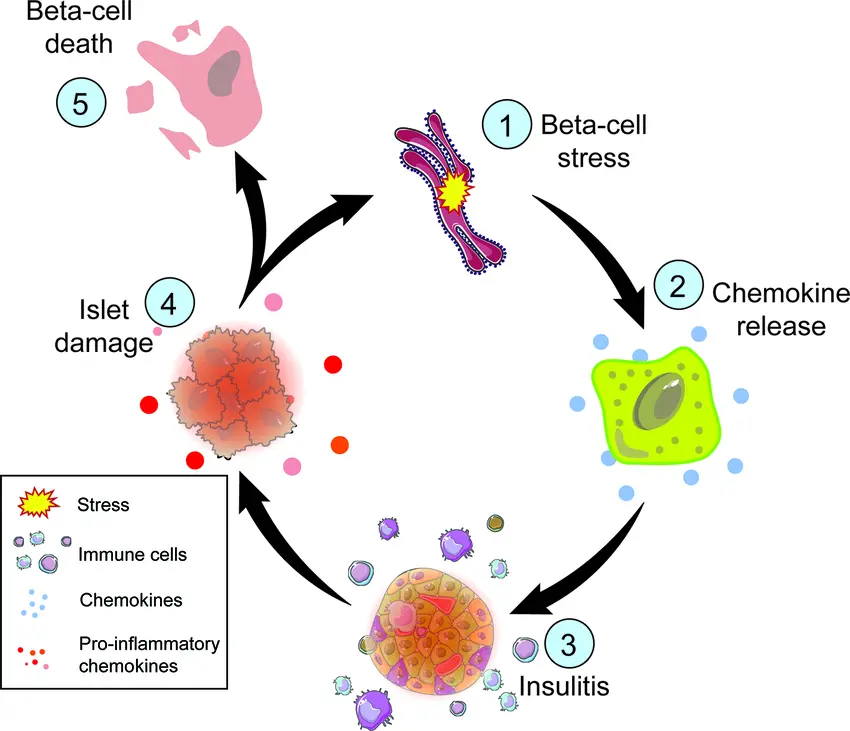
Chronic low-grade inflammation is a hidden but important factor in people with Type 2 diabetes. Cytokines, such as TNF-α and interleukin-1β (IL-1β), are released by the immune system over time and start to attack and disrupt pancreatic function.
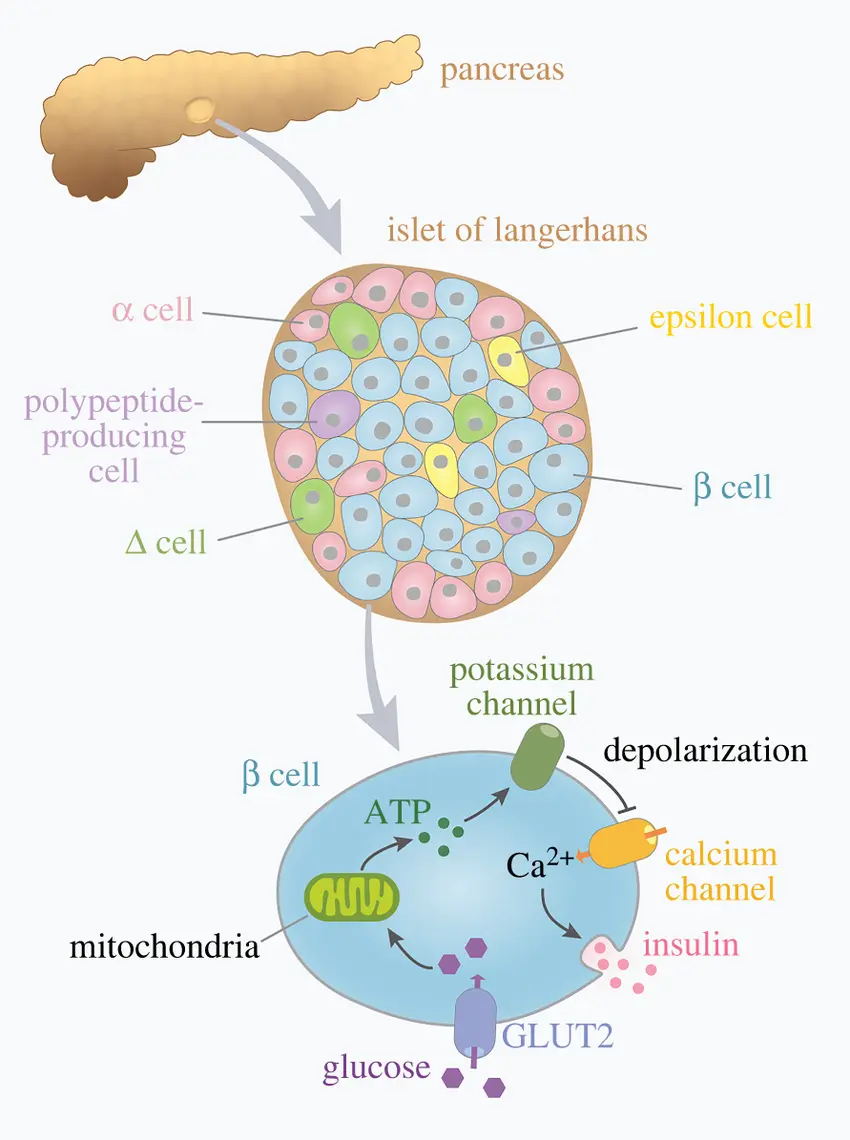
The sensitive environment of the islets of Langerhans, which house beta cells, is harmed by these inflammatory chemicals. This results in:
- Loss of beta-cell mass
- Decreased insulin secretion
- Reduced ability to respond to glucose
- A feedback loop that accelerates disease progression
Protecting beta cells from inflammatory stress is a key goal of next-generation diabetes therapies — and peptides may hold the key.
The Promise of Peptide-Based Therapies
Peptides are short chains of amino acids the very building blocks of proteins that act as signaling molecules in nearly every biological process. Many natural hormones and growth factors are peptides, including insulin itself.
Thanks to advancements in biotechnology and bioengineering, scientists are now able to design synthetic peptides with highly specific biological activity. These therapeutic peptides can:
- Interact with cell surface receptors
- Activate intracellular pathways
- Promote healing, regeneration, or targeted immune responses
In the context of diabetes, certain peptides are being studied for their ability to stimulate insulin secretion, protect beta cells, and promote regeneration.
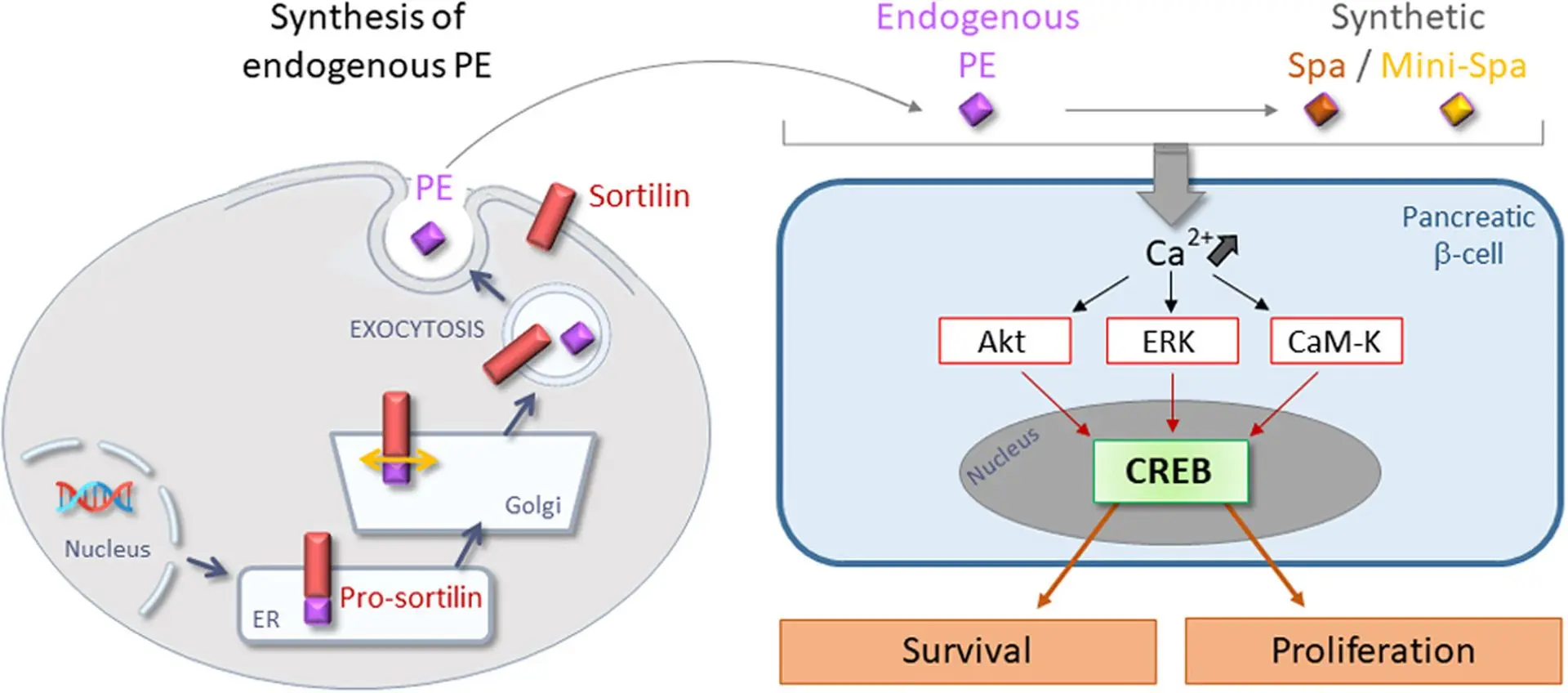
The Discovery of PE: A Dual-Action Natural Peptide
A promising breakthrough centers on a natural peptide known as PE, which is released from a cellular protein called sortilin.
This peptide has two remarkable and clinically relevant properties:
- It enhances insulin secretion — but only in response to elevated glucose levels, reducing the risk of hypoglycemia.
- It protects pancreatic beta cells from destruction caused by inflammatory cytokines like IL-1β.
This combination makes PE especially attractive for safe and targeted therapy, because it works only when needed and preserves vital cell function.
From Nature to Innovation: Spadin and Mini-Spadin
To harness the therapeutic potential of PE, scientists developed two synthetic analogs:
Spadin and Mini-Spadin.
These engineered peptides:
- Mimic the effects of PE in enhancing glucose-dependent insulin secretion
- Protect beta cells under inflammatory stress conditions
- Promote beta-cell survival and resilience at the molecular level
- And notably, Mini-Spadin encourages beta-cell proliferation, pointing to potential regenerative capabilities
This means Mini-Spadin may not only slow the destruction of beta cells — it might actually help the pancreas grow new insulin-producing cells.
⚙️ Mechanism of Action: A Look Inside the Cell
These peptides work by depolarizing the beta cell membrane, leading to a rise in intracellular calcium concentration a key trigger for both insulin release and cell survival signaling.
Specifically, they activate:
- The CaM-Kinase signaling pathway, which protects against apoptosis (cell death)
- The CREB transcription factor, a protein known to regulate genes involved in cell survival, function, and proliferation
This dual action — stimulation and protection — is what makes Spadin and Mini-Spadin unique in the field of peptide therapeutics for diabetes.
💡 Why This Matters for the Future of Diabetes Treatment
Traditional diabetes therapies including metformin, GLP-1 agonists, and insulin injections focus on managing symptoms rather than modifying the disease itself.
They may:
- Improve insulin sensitivity
- Lower blood sugar levels
- Replace lost insulin function
But they do not stop the underlying beta-cell decline or reverse the inflammatory damage to the pancreas. In contrast, peptide-based therapies like PE and its derivatives offer:
✅ Targeted cellular action, working only in the pancreas and only when needed
✅ Safety through glucose-dependency, avoiding unwanted insulin surges
✅ Protection against cytokine-induced stress, slowing disease progression
✅ Potential regeneration of beta cells, offering a truly restorative therapy
This marks a shift toward disease-modifying treatment — a cornerstone of regenerative medicine.
For further information
Here you can find a current, comprehensive article on this topic:
Diabetes: Recent Advances and Future Perspectives