Peptides short chains of amino acids linked by peptide bonds play a crucial role in biological systems, serving as hormones, enzymes, signaling molecules, and therapeutic agents. The ability to synthesize peptides efficiently and accurately is essential for research, drug development, and biotechnological applications. Solid-Phase Peptide Synthesis (SPPS) is a revolutionary technique that has transformed peptide chemistry since its invention in the 1960s, allowing scientists to build peptides step-by-step on an insoluble support. In this blog, we dive deep into the principles, methodology, advantages, challenges, and applications of SPPS.
What is Solid-Phase Peptide Synthesis (SPPS)?
SPPS is a method for chemically assembling peptides while anchored to a solid resin or support. Unlike traditional solution-phase synthesis where the growing peptide is dissolved in liquid, SPPS fixes the initial amino acid to an inert solid support, enabling easy separation of reactants and products by simple filtration and washing steps. This dramatically improves efficiency, purity, and automation potential.
The technique was pioneered by Robert Bruce Merrifield in 1963, who was awarded the Nobel Prize in Chemistry in 1984 for this groundbreaking work. His invention enabled peptide synthesis on a solid resin, revolutionizing the way peptides are made in laboratories worldwide.
Basic Principles of SPPS
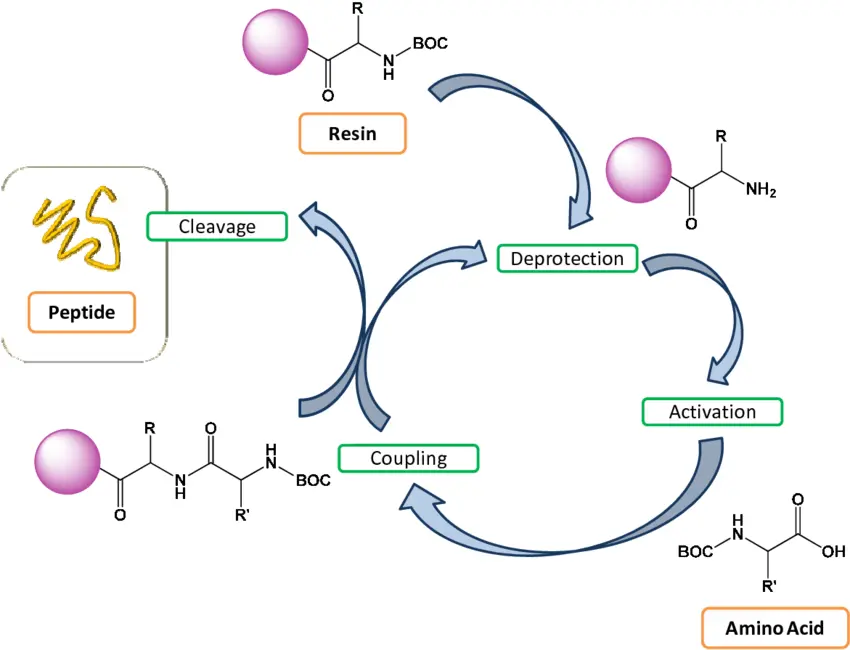
The SPPS process relies on the sequential addition of protected amino acids to a growing peptide chain attached to a solid resin bead. The key features of this process include:
- Solid Support (Resin): The peptide chain begins attached to an insoluble polymer resin, typically polystyrene beads functionalized with reactive groups such as chloromethyl or hydroxymethyl. This allows the peptide to remain immobilized during synthesis.
-
Protected Amino Acids: Each amino acid used in SPPS carries temporary protective groups that block reactive sites to prevent undesired side reactions:
- N-terminal protection: Usually the Fmoc (9-fluorenylmethyloxycarbonyl) or Boc (tert-butyloxycarbonyl) group protects the amino group.
- Side chain protection: Specific protecting groups are attached to amino acid side chains to prevent unwanted reactions.
- Stepwise Coupling: The synthesis proceeds by removing the N-terminal protecting group on the resin-bound peptide and then coupling the next protected amino acid. This cycle repeats until the desired sequence is complete.
- Cleavage and Deprotection: After assembly, the peptide is cleaved from the resin and side chain protecting groups are removed, yielding the free peptide.
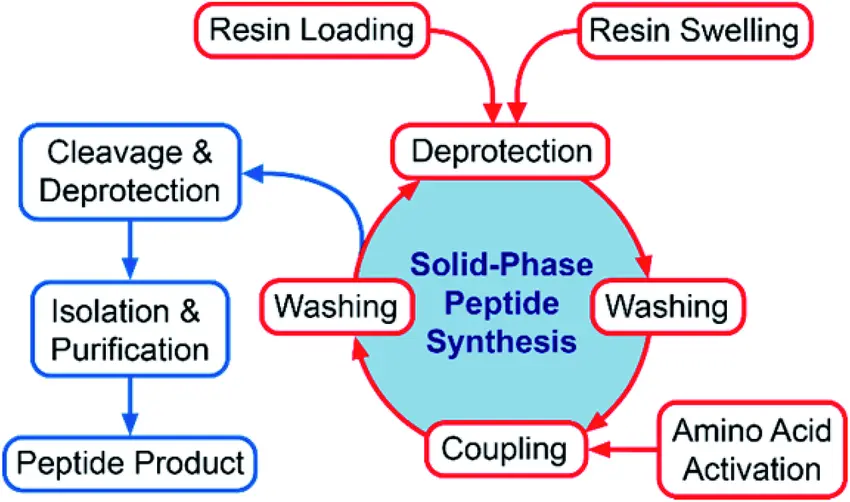
Step-by-Step SPPS Workflow
1. Attachment of the First Amino Acid to the Resin
The synthesis begins by anchoring the C-terminal amino acid of the desired peptide sequence to the solid support. This amino acid is usually pre-activated or coupled via specific chemistry compatible with the resin functional groups.
2. Deprotection
If Fmoc chemistry is used, the N-terminal Fmoc protecting group is removed by a mild base such as piperidine in DMF (dimethylformamide), exposing a free amino group for the next coupling.
3. Coupling
The next protected amino acid is activated (commonly using carbodiimide reagents like DIC or HATU) and then added to the resin, forming a peptide bond with the free amino group.
4. Washing
Excess reagents and byproducts are washed away with solvents such as DMF or dichloromethane (DCM), ensuring a clean environment for the next cycle.
5. Repeat
Steps 2–4 repeat until the full peptide sequence is assembled.
6. Cleavage from Resin
Once the synthesis is complete, the peptide is cleaved from the solid support using strong acid cocktails (trifluoroacetic acid (TFA) mixtures), which also remove side chain protecting groups.
7. Purification and Characterization
The crude peptide is purified typically by high-performance liquid chromatography (HPLC) and characterized by mass spectrometry and other analytical techniques.
Advantages of SPPS
- Efficiency: SPPS eliminates the need for intermediate purification because reagents and byproducts are washed away after each step.
- Automation: Automated peptide synthesizers can perform hundreds of coupling cycles rapidly, enabling fast production of complex peptides.
- High Purity: Sequential coupling and washing reduce side reactions and impurities.
- Versatility: SPPS can be adapted to synthesize peptides of varying lengths and complexities, including modified peptides and peptidomimetics.
- Scalability: From milligram to multi-gram scale, SPPS protocols can be adjusted for research or industrial production.
Applications of SPPS
SPPS has widespread applications across research and industry:
-
Protein Structure and Function Studies: Synthetic peptides allow scientists to study protein domains, active sites, and protein-protein interactions.
- Diagnostics: Peptide probes and biomarkers are synthesized via SPPS for disease detection.
- Material Science: Peptide-based hydrogels and biomaterials rely on synthetic peptides produced by SPPS.
Innovations and Future Directions
Recent advances are pushing SPPS further:
- Microwave-Assisted SPPS: Accelerates coupling and deprotection steps, reducing synthesis times.
- Automated High-Throughput Synthesizers: Enable parallel synthesis of peptide libraries for drug discovery.
- Green Chemistry Approaches: Efforts to reduce solvent use and waste generation during synthesis.
- On-Resin Cyclization and Modifications: Allow for the synthesis of cyclic peptides and peptides with post-translational modifications.
Solid-Phase Peptide Synthesis remains the cornerstone technique for peptide production worldwide. Its efficiency, versatility, and compatibility with automation have made it indispensable for biochemistry, pharmaceutical development, and biotechnology. As innovations continue to optimize SPPS, including faster synthesis protocols and greener chemistries, this technique will keep fueling advances in peptide science and therapeutic discovery for years to come.